This video shows the initial testing of a medium-frequency pulse-detonation burner performed at our pilot-scale biomass gasification test facility located at UC Riverside. Typically, we are operating for 10-minute intervals, watching particularly for hot-spots and for any firing instabilities that develop over time.
The firing rate for this direct-spray-cooled pulse-detonation burner can go a lot higher. During the initial testing, I accidentally swung the dial to turn up the cycle frequency, and although we had not planned for high-frequency testing, the firing rate went right off the chart before I could cut the inputs. Therefore, at the very least, we can confirm the work of Ken Matsuoka’s team at Nagoya University, where this advanced technique of using atomized-water to purge combustion products was developed and demonstrated at firing rates up to 350-Hz.
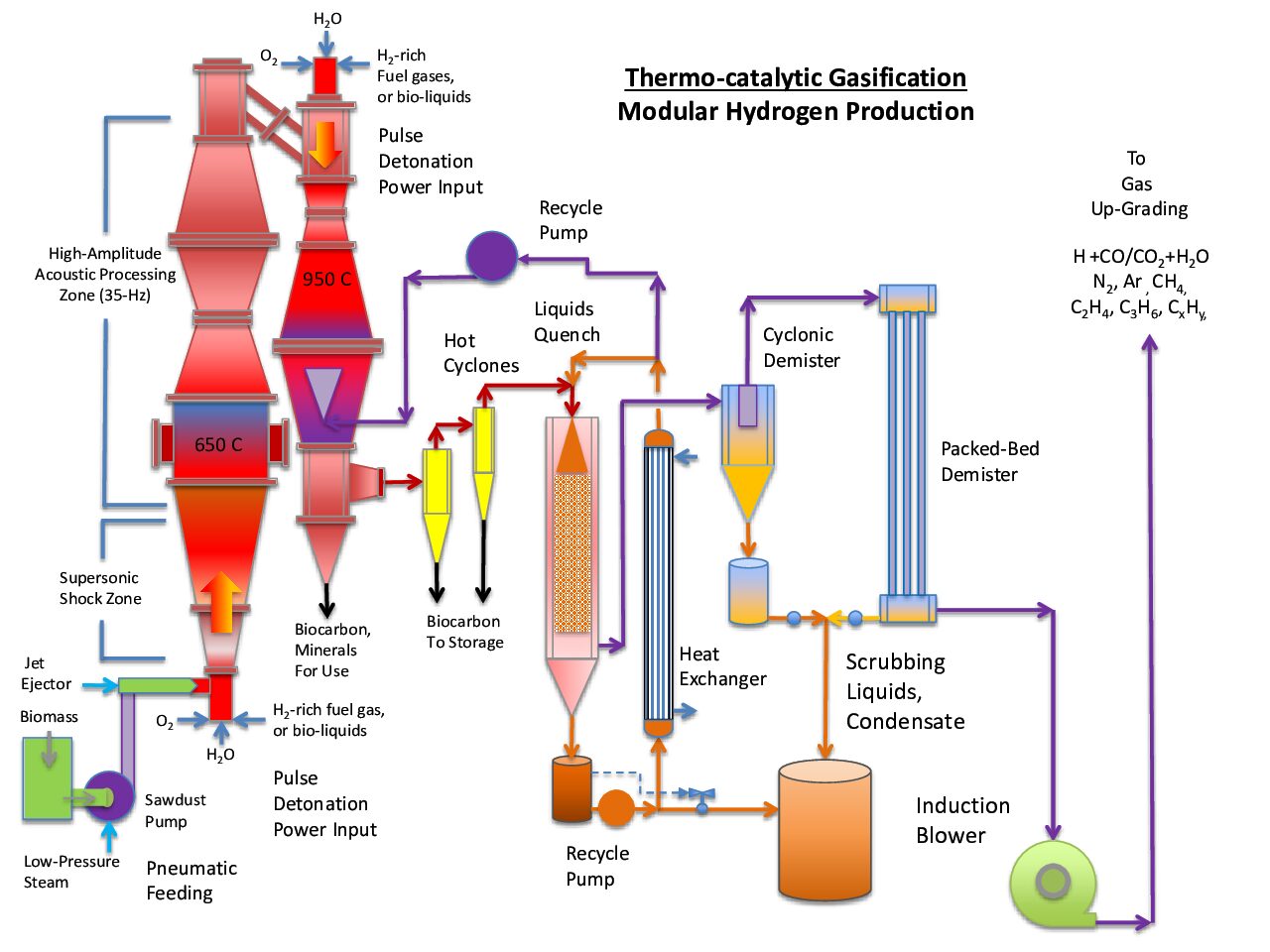
I suspect that we will select a firing rate somewhere between 20-Hz and 40-Hz; although, we are just now evaluating the performance of the direct-spray-cooled detonation burners that offer low-cost and operating simplicity when compared to alternative methods used for thermochemical process intensification, which is our goal relative to organic gasification of municipals wastes and the production of renewable hydrogen fuel.
The power generated by repetitive shockwaves is rather astounding; the clicking sound you hear from your computer is not indicative of power level one feels in proximity to the pulse-detonation discharges. Getting within about four feet of the discharge — just on the side, not in front — starts to feel dangerous to one’s grey matter. When applied to organic gasification at high temperature, the process improvements will be significant.
Materials need to be “worked” during processing; much the way metals are hardened by folding and hammering to make great swords, solids and gases must be “worked” to enhance both the heat and mass transfer functions; shockwaves cause the micronizing of friable solids and promote the rapid mixing of gases and solids to intensify the gasification process, without the need for fluidized bed materials that otherwise physically impede the desired thermochemical interactions, for example, between water-vapor and micronized carbonaceous solids, the renewable energy source used to react with water (H2O) to liberate ultra-clean hydrogen fuel (H2).